Eisai applies for PMDA approval for subcutaneous Leqembi in Japan
- Last update: 4 days ago
- 2 min read
- 17 Views
- HEALTH
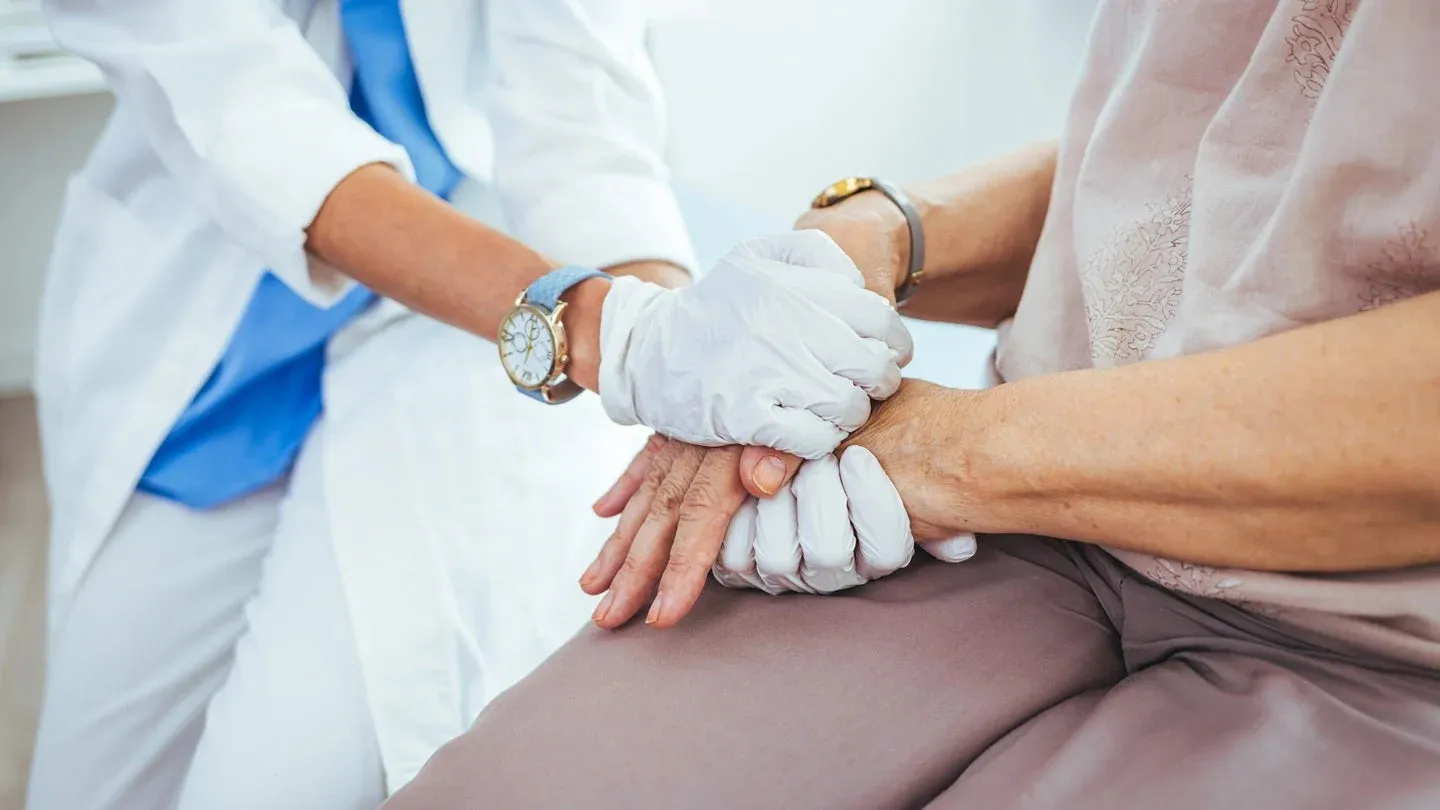
Eisai has filed a new drug application with Japans Pharmaceuticals and Medical Devices Agency (PMDA) for the subcutaneous (SC) version of Leqembi (lecanemab), known as SC-AI, intended for patients with early-stage Alzheimers disease. This formulation introduces an alternative method of administration.
The submission is supported by results from multiple sub-studies investigating SC administration of lecanemab within the Phase III Clarity AD open-label extension. These studies followed participants for 18 months after the core trial, focusing on individuals with mild cognitive impairment linked to early Alzheimers dementia. Findings indicated that a weekly SC-AI 500mg dose delivered via autoinjector produced exposure levels comparable to biweekly intravenous dosing, with similar clinical and biomarker outcomes.
The safety profile for the SC route was largely consistent with intravenous administration, with fewer than 2% of participants experiencing systemic injection or infusion-related reactions. If approved, patients could self-administer two 250mg SC-AI injections at home once a week, providing an alternative to the current biweekly hospital-based intravenous regimen. Each autoinjector delivers its dose in approximately 15 seconds.
Approval of SC-AI could broaden treatment options, allowing both patients and caregivers to manage therapy at home while reducing the healthcare resources needed for intravenous administration, such as nurse monitoring and preparation, thereby simplifying Alzheimers care pathways.
Leqembi is already approved in 51 countries and regions, with ongoing regulatory review in nine additional locations. Eisai manages global development and regulatory efforts and collaborates with Biogen on commercialisation and promotion. In November 2025, the UK Medicines and Healthcare products Regulatory Agency (MHRA) approved Leqembi for intravenous maintenance dosing every four weeks for early Alzheimers disease.
Author: Sophia Brooks
Share

Top 4 Frozen Foods for Heart Health Recommended by a Cardiologist and a Nutrition Researcher
1 hours ago 3 min read HEALTH

Top Aerobic Exercise Tip for Longevity Shared by Fitness Experts
1 hours ago 3 min read HEALTH

New Study Reveals That This Popular Diet May Make It More Difficult for You to Have a Bowel Movement
1 hours ago 3 min read HEALTH

Health Rounds: Study finds shingles vaccine decreases risk of dementia-related death
3 hours ago 2 min read HEALTH

Newly FDA Approved Glasses Can Help Slow Down Your Child's Myopia Progression
4 hours ago 2 min read HEALTH

Atraverse receives FDA approval for HOTWIRE transseptal access system
5 hours ago 2 min read HEALTH

FDA warns of 7 deaths and more than 700 injuries linked to faulty glucose monitors
6 hours ago 2 min read HEALTH
The FDA confirmed 10 children died after receiving COVID vaccines. SAN verified the information.
6 hours ago 3 min read HEALTH

Make sure to mark your calendars for these Tricare and other health benefits deadlines
7 hours ago 3 min read HEALTH

Cardiologists Say These 2 Tips Can Help During the 'Worst Time of the Year' for Heart Health
8 hours ago 2 min read HEALTH