Atraverse receives FDA approval for HOTWIRE transseptal access system
- Last update: 56 minutes ago
- 2 min read
- 489 Views
- HEALTH
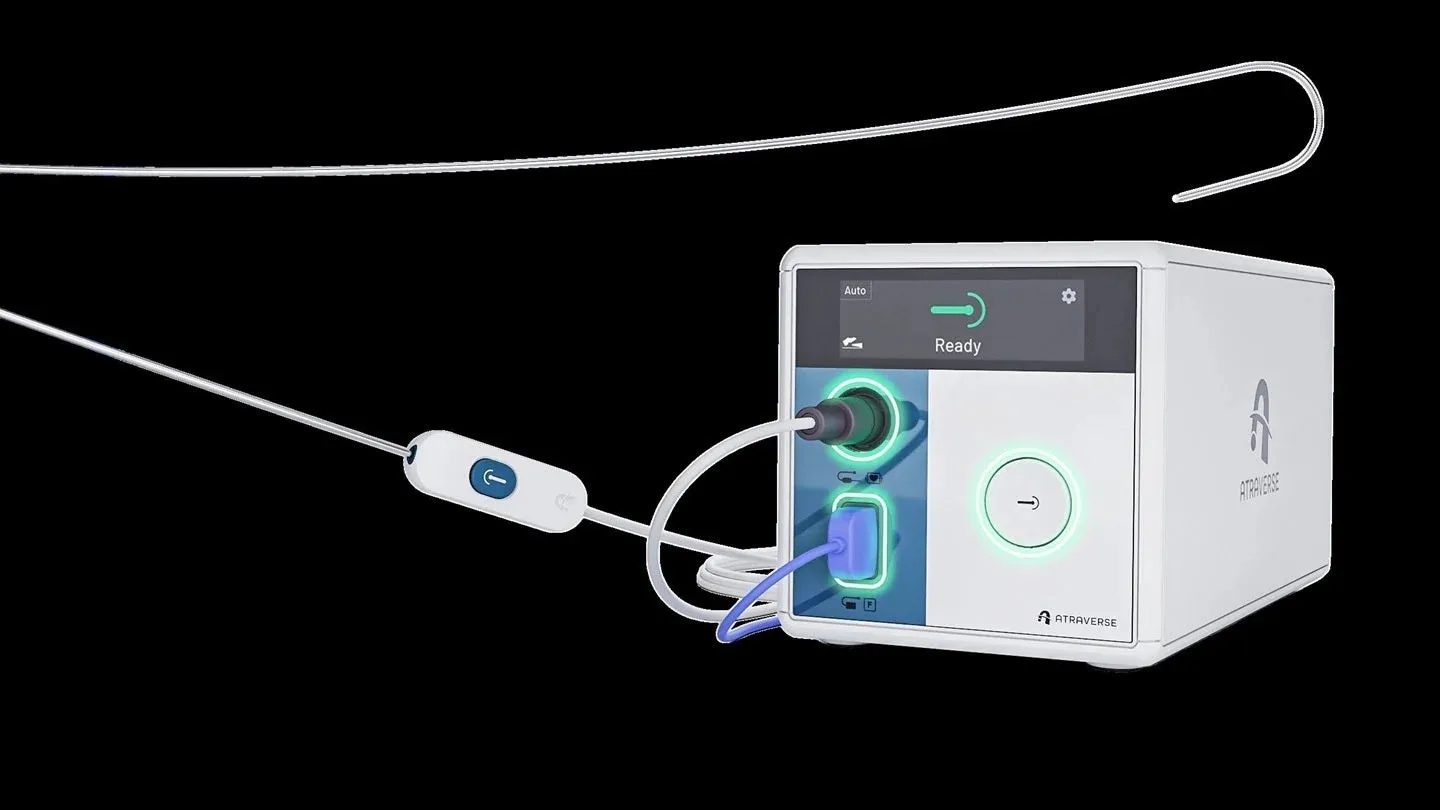
Atraverse Medical has announced that its fully integrated HOTWIRE transseptal access platform has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This innovative system includes the HOTWIRE RF generator, recognized as the first left-heart access device using impedance-guided technology that automatically halts energy delivery once transseptal crossing is achieved. The design is intended to minimize unnecessary radiofrequency (RF) exposure in the left atrium and gives clinicians precise control over energy activation directly within the sterile field.
The platform also incorporates the zero-exchange HOTWIRE RF guidewire, compatible with a broad selection of sheaths. Its specially engineered tip is reported to enhance echocardiographic visibility by 25%, and the reinforced core wire with polymer coating adds durability and reliability. The HOTWIRE RF guidewire, which gained FDA clearance in May 2024, has already been utilized in nearly 2,000 clinical cases.
Dr. Devi Nair, cardiac electrophysiology director at St. Bernard's Heart and Vascular Center, conducted the initial procedures with the complete HOTWIRE system. Dr. Nair commented: "Achieving safe and precise left atrial access remains one of the most critical steps in electrophysiology and structural heart procedures. In my experience, the HOTWIRE platform offers exceptional control, accuracy, and procedural efficiency, significantly improving the transseptal workflow. I am eager to present these findings at the AF Symposium, as this technology represents a meaningful advancement in transseptal access."
Eric Sauter, COO, co-inventor, and co-founder of Atraverse Medical, stated: "Obtaining FDA clearance for the HOTWIRE system marks a major milestone for Atraverse, made possible by the dedication, creativity, and technical skill of our development team. After three years of focused development, our fully integrated system and purpose-built RF generator provide a seamless solution to a longstanding clinical challenge, creating new opportunities for both commercial growth and improved patient outcomes."
Author: Lucas Grant
Share

Newly FDA Approved Glasses Can Help Slow Down Your Child's Myopia Progression
10 minutes ago 2 min read HEALTH

Atraverse receives FDA approval for HOTWIRE transseptal access system
56 minutes ago 2 min read HEALTH

FDA warns of 7 deaths and more than 700 injuries linked to faulty glucose monitors
2 hours ago 2 min read HEALTH
The FDA confirmed 10 children died after receiving COVID vaccines. SAN verified the information.
2 hours ago 3 min read HEALTH

Make sure to mark your calendars for these Tricare and other health benefits deadlines
2 hours ago 3 min read HEALTH

Cardiologists Say These 2 Tips Can Help During the 'Worst Time of the Year' for Heart Health
3 hours ago 2 min read HEALTH
US vape products recalled due to possible contamination: Urgent advice to seek medical help
6 hours ago 2 min read HEALTH

CDC advisers postpone important decision on hepatitis B vaccination during tense meeting
7 hours ago 3 min read HEALTH

RFK Jr. takes action after school vaccinates child without permission
17 hours ago 2 min read HEALTH

CDC cautions travelers about rabies in these 2 countries
18 hours ago 2 min read HEALTH