Important information about hepatitis B vaccination for infants before CDC vaccine panel meeting
- Last update: 2 days ago
- 3 min read
- 106 Views
- HEALTH
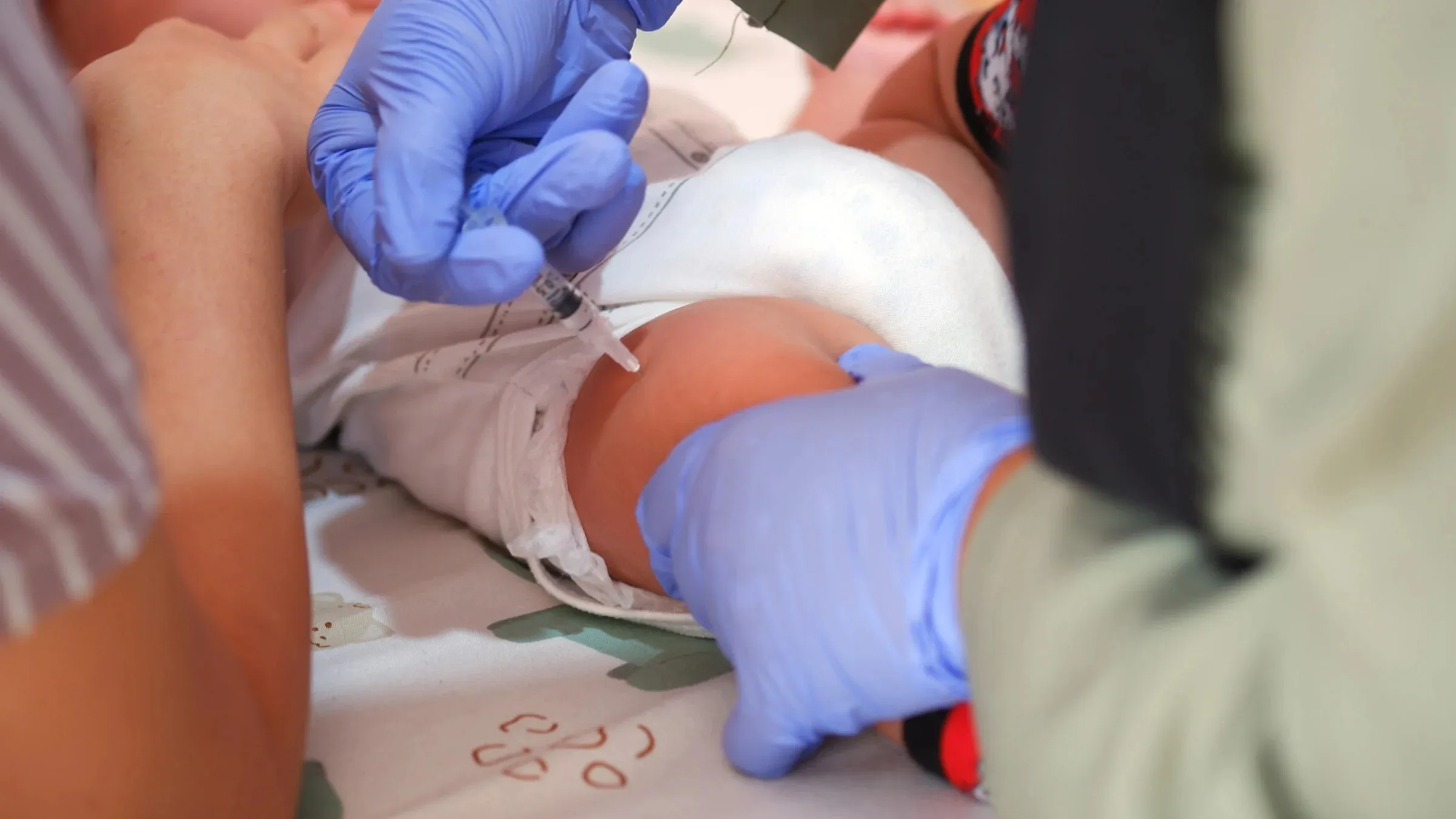
The Centers for Disease Control and Preventions (CDC) vaccine advisory committee is expected to review potential updates to the childhood immunization schedule, including the hepatitis B vaccine administered to newborns. The Advisory Committee on Immunization Practices (ACIP) will convene Thursday and Friday, with a draft agenda noting a discussion on hepatitis B vaccination and possible votes on related recommendations.
Although the specific items for vote are not disclosed, statements from Health and Human Services Secretary Robert F. Kennedy Jr. and ACIP members suggest that the universal birth dose of the hepatitis B vaccine may be reconsidered. ACIP could vote to delay or remove the recommendation for the dose given at birth.
Public health experts emphasize that the hepatitis B vaccine is safe and effective. Vaccinating infants at birth has dramatically reduced the incidence of hepatitis B in children. Dr. Fiona Havers, a former CDC official, explained that nearly 99% of childhood hepatitis B cases have been prevented due to the birth dose. She added that infection during infancy often leads to lifelong disease, increasing risks of liver failure, cancer, and death.
The hepatitis B vaccine is typically administered in a three-dose series: the first dose within 24 hours of birth, the second between 1 and 2 months, and the third between 6 and 18 months. The vaccine is recommended for all infants, children, and adults under 60, with older adults at risk also advised to receive it.
Initially, hepatitis B vaccination targeted older children and adults, with newborns only vaccinated if identified as high-risk. However, screening missed many cases. Dr. Susan Wang, a former CDC hepatitis B expert, stated that decades of data support the safety and effectiveness of giving the vaccine within 24 hours of birth. ACIP adopted this approach in 1991 to curb hepatitis B transmission in the United States.
Why the Birth Dose Remains Critical
Dr. William Schaffner, a professor of preventive medicine, explained that infants born to hepatitis B-positive mothers face an 85% risk of infection at birth, and a 90% chance of developing chronic hepatitis B if infected. Chronic infection can lead to liver disease, cirrhosis, and cancer. Prior to the 1991 recommendation, approximately 20,000 infants were infected annually from their mothers; now, fewer than 20 cases occur each year.
Schaffner warns that delaying the birth dose could result in children missing vaccination entirely, increasing infection risk and perpetuating transmission within the population.
Claims Challenging the Vaccine
Some public figures, including RFK Jr., have questioned the necessity of the birth dose, suggesting hepatitis B is primarily transmitted through sexual contact or needle use. However, experts note that relying on testing only misses cases and leaves infants unprotected. Dr. Wang highlighted that the vaccine is safe, cost-effective, and highly beneficial, protecting newborns both from maternal transmission and from other potential exposures.
Potential Implications of Changing the Recommendation
Experts compare removing the universal birth dose to removing a seatbelt in a car: it eliminates a critical protection against preventable harm. Vaccinating infants early acts as post-exposure prophylaxis and prevents chronic infections. Delaying the vaccine could maintain hepatitis B circulation in future generations and lead to increased healthcare costs if vaccines are no longer universally recommended.
ACIP guidance also influences insurance coverage and the federally funded Vaccines for Children program. Any change to the recommendation could result in out-of-pocket expenses for families and reduced vaccine accessibility for eligible children.
Author: Gavin Porter
Share

Health Rounds: Study finds shingles vaccine decreases risk of dementia-related death
15 minutes ago 2 min read HEALTH

Newly FDA Approved Glasses Can Help Slow Down Your Child's Myopia Progression
1 hours ago 2 min read HEALTH

Atraverse receives FDA approval for HOTWIRE transseptal access system
2 hours ago 2 min read HEALTH

FDA warns of 7 deaths and more than 700 injuries linked to faulty glucose monitors
4 hours ago 2 min read HEALTH
The FDA confirmed 10 children died after receiving COVID vaccines. SAN verified the information.
4 hours ago 3 min read HEALTH

Make sure to mark your calendars for these Tricare and other health benefits deadlines
4 hours ago 3 min read HEALTH

Cardiologists Say These 2 Tips Can Help During the 'Worst Time of the Year' for Heart Health
5 hours ago 2 min read HEALTH
US vape products recalled due to possible contamination: Urgent advice to seek medical help
8 hours ago 2 min read HEALTH

CDC advisers postpone important decision on hepatitis B vaccination during tense meeting
9 hours ago 3 min read HEALTH

RFK Jr. takes action after school vaccinates child without permission
19 hours ago 2 min read HEALTH