Recall of Glucose Monitors for Diabetes Linked to 7 Deaths and Over 700 Injuries
- Last update: 6 hours ago
- 2 min read
- 490 Views
- WORLD
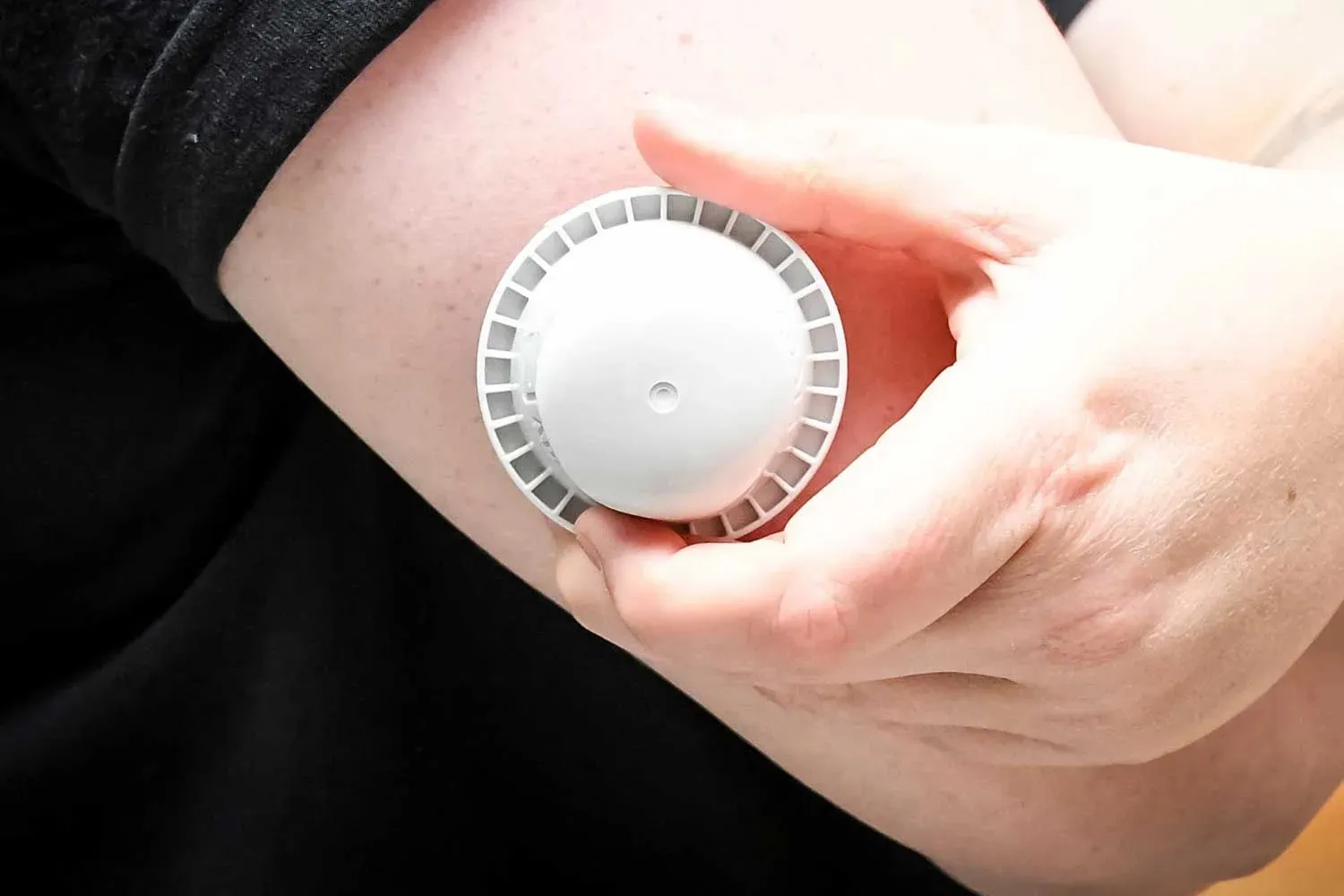
Abbott Diabetes Care has issued an urgent recall of certain glucose monitoring devices after seven deaths and hundreds of reported injuries. Patients are advised to immediately stop using affected FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors.
The U.S. Food and Drug Administration (FDA) confirmed on Dec. 2 that Abbott identified faulty sensors during internal testing, which may give inaccurate low glucose readings. These malfunctions could be linked to more than 700 adverse events worldwide, including 57 in the United States.
"Abbott has initiated a correction for specific FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors after discovering that some may deliver incorrect low glucose readings," the company stated. Only these two models are involved; other Libre devices are unaffected.
Patients can check if their sensor is affected by visiting www.FreeStyleCheck.com. Abbott and the FDA stress that affected users should discontinue use and rely on a traditional blood glucose meter or the built-in meter on the FreeStyle Libre 3 Reader for treatment decisions if sensor readings are inconsistent with symptoms.
The company reported 736 serious adverse events potentially related to these monitors, including seven deaths outside the U.S. The FDA classified the issue as "potentially high-risk."
Approximately 3 million Libre 3 and Libre 3 Plus sensors in the U.S. are part of this recall. About half of these may have already been used or expired. Users should check their devices model number and unique device identifier against the recall list:
- FreeStyle Libre 3 sensors: model numbers 72081-01 and 72080-01; UDIs 00357599818005 and 00357599819002
- FreeStyle Libre 3 Plus sensors: model numbers 78768-01 and 78769-01; UDIs 00357599844011 and 00357599843014
Abbott will provide replacements for affected sensors at no cost. The company has identified the cause of the issue and corrected it, assuring that production continues to meet replacement and new order demands without major delays.
According to the Mayo Clinic, glucose meters are vital for people with diabetes to monitor blood sugar levels influenced by diet, exercise, medications, and stress. Abbott warns that inaccurate low glucose readings can lead to harmful treatment decisions, including excessive carbohydrate intake or skipped insulin doses, posing serious health risks.
Author: Natalie Monroe
Share

Serious injury to pedestrian in Shipley accident
51 seconds ago 1 min read WORLD

U.K. and Norway agree to collaborate in tracking Russian submarines in North Atlantic
1 minutes ago 2 min read WORLD

Radio 1 newsreader fulfills CBeebies dream with royal support
4 minutes ago 2 min read WORLD

Confirmation: Nanotyrannus was a separate species, not a young T. rex
4 minutes ago 3 min read WORLD

Is It Possible to Capture Images of Alien Earths? This Newly Discovered Object Could Provide the Answer
6 minutes ago 2 min read WORLD

City hospital urges wearing masks during flu outbreak
7 minutes ago 1 min read WORLD

New clashes in eastern Congo put at risk 'historic' peace agreement mediated by Trump
9 minutes ago 2 min read WORLD

‘Continuous oil deliveries’: Main points from Putin-Modi discussions in Delhi
10 minutes ago 4 min read WORLD

IPCC chief urges UN scientists to be 'clear' on human impact to combat climate denial
11 minutes ago 2 min read WORLD

Crash and car fire lead to closure of busy road
11 minutes ago 1 min read WORLD