Understanding the hepatitis B vaccine and the reasons behind Trump officials targeting it
- Last update: 1 days ago
- 3 min read
- 145 Views
- POLITICS

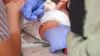
NEW YORK This week, a federal advisory committee on vaccines is expected to review whether newborns should continue receiving the hepatitis B vaccine, the first vaccine known to prevent cancer. Current U.S. health guidance recommends that all newborns receive the shot within their first 24 hours, but the committee led by Health Secretary Robert F. Kennedy Jr. may propose changes, diverging from longstanding public health advice.
Details of the committees considerations are not fully disclosed. However, the American Academy of Pediatrics continues to support the birth dose, citing its life-saving benefits, according to Dr. Sean OLeary.
Understanding Hepatitis B
Hepatitis B is a liver infection that usually resolves in adults within six months, but in infants and children, it can become chronic, potentially causing liver failure, cirrhosis, or cancer. Adults typically contract the virus through sexual contact or sharing needles, while infants can be infected at birth from their mothers. About 90% of infected infants develop long-term infections. In the U.S., roughly 2.4 million people carry hepatitis B, with nearly half unaware of their infection, according to CDC data.
History of the Vaccine
Dr. Baruch Blumberg identified the virus in 1965, earning a Nobel Prize and paving the way for testing and vaccine development. The first hepatitis B vaccine was licensed in the U.S. in 1981.
Newborn Vaccination Guidelines
The Advisory Committee on Immunization Practices (ACIP), a federal expert panel, has influenced national vaccine policy for decades. In 1991, the committee recommended giving the first hepatitis B dose at birth. Today, guidance advises vaccination within 24 hours for medically stable infants weighing at least 4.4 pounds (2 kg), with follow-up doses at one month and six months.
Vaccinating at birth addresses cases missed during prenatal screening and prevents infections from household contacts. The strategy has dramatically reduced pediatric hepatitis B cases in the U.S., from about 18,000 annually to 2,200 over three decades. A recent review of over 400 studies by the Vaccine Integrity Project confirmed the birth dose is safe and pivotal in lowering pediatric infections.
Reevaluation of the Birth Dose
Earlier this year, Kennedy replaced all 17 ACIP members with a new panel including voices critical of vaccines. Concerns have been raised about vaccinating infants so soon after birth. Some committee members question whether the early dose addresses an adult problem and whether parents are fully informed. The vote on the recommendation has been postponed but is scheduled for this week.
Potential Consequences of Delaying the Shot
The impact of delaying vaccination is uncertain, but research suggests that postponing the first dose to two months could lead to at least 1,400 new infections and 480 deaths among children. Delays beyond that could increase the toll. ACIPs recommendations mainly influence government-funded programs like Vaccines for Children, so hospitals may continue the current practice regardless, but public confusion and concern are likely.
Responses from Public Health Leaders
Medical and public health organizations, as well as some state officials, oppose changing the recommendation. A coalition of Northeastern state leaders has pledged to continue advocating for the birth dose within 24 hours. U.S. Senator Patty Murray called for Kennedy to testify before Congress, warning that ending the long-standing guidance could put infants lives at risk.
Author: Grace Ellison
Share

Can COVID-19 vaccines cause early miscarriage? No.
1 days ago 2 min read POLITICS

Twelve ex-Commissioners of the US FDA express grave concerns over agency's vaccine policy shift, according to NEJM
1 days ago 2 min read BUSINESS

Twelve former FDA leaders criticize current FDA vaccine chief's claims
1 days ago 2 min read POLITICS

US authorities are examining the Hepatitis B vaccine for newborns
1 days ago 2 min read POLITICS

FDA's statements on COVID-19 vaccine safety lack reliable data support and may impede vaccine accessibility
1 days ago 3 min read HEALTH
Former FDA commissioners criticize Trump administration's vaccine plan
1 days ago 2 min read POLITICS

RFK Jr.'s panel may soon change the hepatitis B vaccine. What you should be aware of
1 days ago 4 min read POLITICS

RFK Jr.’s advisory panel could revise recommendations on childhood vaccines
1 days ago 4 min read HEALTH
Important information about hepatitis B vaccination for infants before CDC vaccine panel meeting
2 days ago 3 min read HEALTH

Health secretary assures there is sufficient supply of flu vaccine
2 days ago 2 min read WORLD